Metastable Impact Electron Spectroscopy
MIES is a very surface-sensitive investigation method in which metastable noble gas atoms of thermal energy interact with the surface of solids by emitting an electron. The kinetic energy of the metastable noble gas atoms, in our case helium, is approx. 58 meV. This energy is neither sufficient to penetrate the sample surface nor to cause mechanical sputtering effects. All processes take place at a distance of approx. 3-10 Å from the surface. When the metastable helium atoms hit the surface, they are partially excited and return to the ground state. The probability of this is between 0.1 and 0.6 depending on the work function of the solid surface. During the transition to the ground state, electrons can be emitted from the sample surface, the energy distribution of which provides information about the electronic structure of the surface. In order to interpret the measurement results correctly, the mechanisms that cause the electrons to be emitted and their probabilities must be known. The mechanisms are as follows.
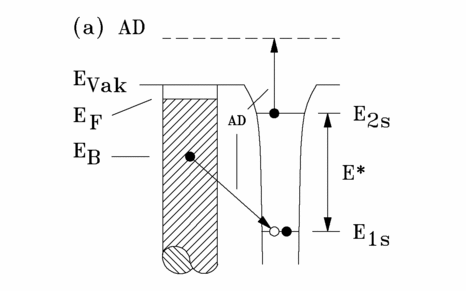
Auger Deexcitation (AD)
The Auger excitation process (schematic diagram in Fig. 3.2) is also known as surface Penning ionization. It takes place largely independently of the work function when a metastable helium atom strikes a solid surface of an insulator, semiconductor or metal with a transition rate in the range of 3*1014 s-1 takes place.
An electron from an occupied state of the sample surface with binding energy EB occupies the unoccupied 1s state of the He*-atom. In an interatomic Auger process, the energy EB - E1s is transferred to the 2s electron, which is emitted. The following energy balance results from Fig. 3.2:
If the excitation energy E* of the metastable helium atoms by hw, equation (3.10) takes the form of the equation for UPS. If this difference in excitation energies is compensated by superimposing the spectra in such a way that the Fermi levels match, MIES spectra resulting from an Auger excitation process and UPS spectra can be compared with each other. It should be noted, however, that the Auger excitation spectrum only contains information about the electronic structure of the surface, whereas in the UPS spectrum this information is superimposed by bulk properties.
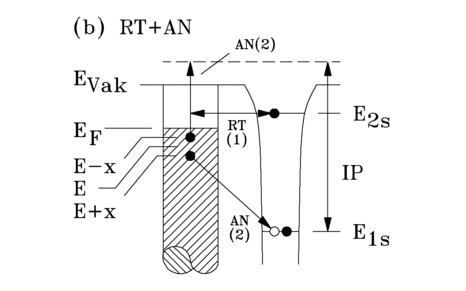
Resonance transfer and Auger neutralization (RT + AN)
Resonance transfer with subsequent Auger neutralization is only possible if the 2s electron of the He* atoms can tunnel into an unoccupied state of the solid surface. This process takes place on metal and semiconductor surfaces, but not on insulators, as the valence band of an insulator lies below the 2s level of the He*. Fig. 3.3 shows a schematic representation of these two processes.
The work function of the solid surface must be greater than the asymptotic ionization potential (IP*) of the metastable helium. This can be understood by the larger overlap of the excited orbital of the He* with free states above the Fermi level, compared to the overlap of the ground state orbital with occupied states of the metal conduction band. Therefore, the resonance transfer process takes place at a greater distance in front of the surface than the Auger excitation process. With a sufficiently large work function (approx. 3.8 eV), resonance transfer with subsequent Auger neutralization therefore generally predominates. During resonance transfer, the 2s electron of the He* tunnels into an unoccupied surface state. The resulting positively charged He ion (He+) is neutralized by a solid-state electron. During this neutralization process, the transition energy is transferred without radiation to another electron of the solid, which is emitted. The emitted Auger electrons originate from the outermost atomic layer, since the transition rate (1*1016 s-1) of the Auger neutralization process essentially depends on the overlap between occupied states of the solid surface and the unoccupied 1s state of the helium ion. The energy distribution N(E) of the emitted electrons is described by a self-folding of the spectroscoped states.
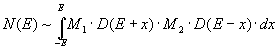
D(E) denotes the spectroscopic surface density of states (SDOS), M1 and M2 denote the transition matrix elements of the electron transitions (labeled RT(1) and AN(2) in Fig. 3.3). The spectra consist of a convolution, since two solid-state electrons with independent transition matrix elements (M1, M2) are always involved in the RT + AN process. Because of this folding, relatively structureless spectra are obtained compared to the AD spectra. The convolution of which the spectra consist can be approximately unfolded by differentiation.
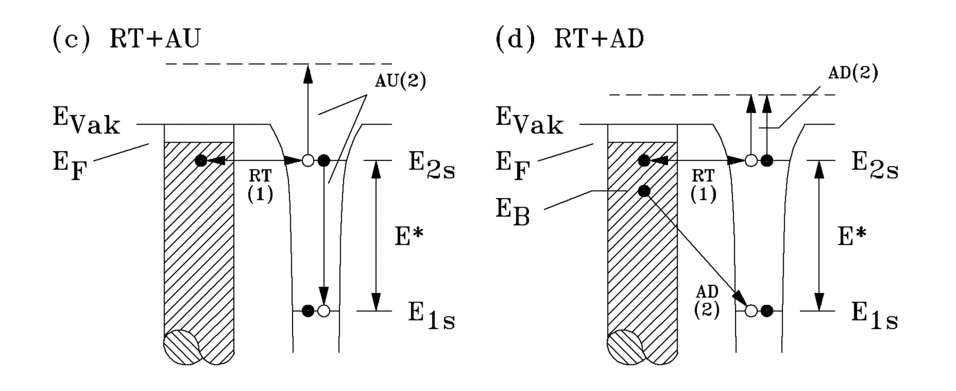
Resonant transfer (RT) with autodetachment (AU) or Augerdeexcitation (AD)
If the work function is very small (< 2.2 eV), an electron can resonate from an occupied solid state of a metal or semiconductor into the single occupied (2s) state of the He* state (RT). This cannot happen with insulators, as the upper edge of the valence band is much lower than the He*(2s) state. The resulting excited helium ion He-*(1s2s2) can be converted to the ground state by autodetachment (AU) or Auger decitation (AD) (Fig. 3.4).
RT + AU
A 2s electron of the He-* passes into the free (1s) state in an intraatomic Auger process. The transition energy is transferred to the second 2s electron, which is emitted. In the spectrum, this process leads to a sharp structure near the Fermi level, as this process can only take place when the 2s level of the He-* is below the Fermi level of the solid.
RT + AD
The free (1s) state is occupied by an electron from an occupied state in the solid in an interatomic Auger process. The transition energy is transferred to the two 2s electrons of the He-*, which are emitted.
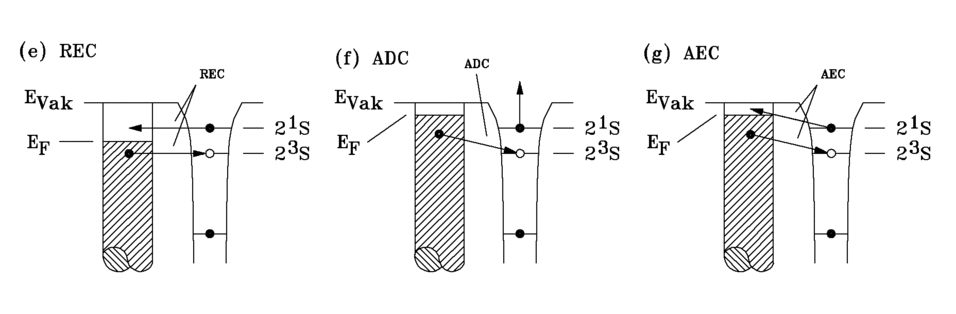
Singlet-triplet conversion
The processes described so far are independent of the spin state of helium. They take place both in the singlet (21S0), as well as in the triplet state (23S1) take place. The ratio of singlet to triplet was determined by He*-argon collisions. It is 1:7 for our source. The singlet state has an excitation energy 0.8 eV higher than the triplet state. In metallic solids, the singlet state is converted to the triplet state before the processes mentioned above take place. The following mechanisms lead to this conversion (Fig. 3.5).
REC model (Resonant Excitation Conversion model)
If the Fermi level of the solid is between the singlet level and the triplet level, the21S electronof the He*( in the singlet state) can tunnel into an unoccupied state in the solid, whereupon the triplet state of the resulting He+ is resonantly occupied by an electron from the solid.
ADC model (Auger Deexcitation Conversion model)
This model applies to the case where the Fermi level of the solid is above the (21S) state of He*. An electron from the solid occupies the (23S) state in the He*, so that a He-*(1s2s2S) ion is formed. The transition energy is transferred to the electron in the (21S) state, which is emitted. The He-*(1s2s2S) ion thus changes to the He*-(23S) state.
AEC model (Auger Excitation Conversion model)
As in (f), the Fermi level of the solid is above the (21S) state of He*. The (23S) state is occupied by an electron from the solid. The transition energy is transferred in an intramolecular Auger process to the electron in the (21S) state, which then occupies an unoccupied state in the solid. In the case of insulators, no conversion occurs because the upper edge of the valence band is much lower than the He*(2s) level, so that the conversion processes cannot take place.
For further information see here.