Solid/liquid interfaces occur in almost all chemical, biological and geological systems. For example, the electrode/electrolyte interface plays a decisive role in chemical reactions such as those that occur on catalyst surfaces in fuel cells. In general, in addition to the electronic and chemical properties of electrode surfaces, it is the atomic or molecular properties of adsorbates at these interfaces that have a decisive influence on the mode of action and efficiency of catalysts (electrodes). However, processes such as those that take place in fuel cells, batteries or during the corrosion of metals are far too complex to be understood without well-defined model systems. For this reason, metal single crystals or thin metal films with a specific preferred orientation are used and these are prepared and studied in-situ. We use two complementary methods for this purpose: Electrochemical scanning tunneling microscopy (ECSTM), and sum frequency vibrational spectroscopy (SFG).
We investigate the following systems:
- Methanol oxidation and CO poisoning on Pt(hkl) surfaces
- Electrocatalytic properties of thin Pt films
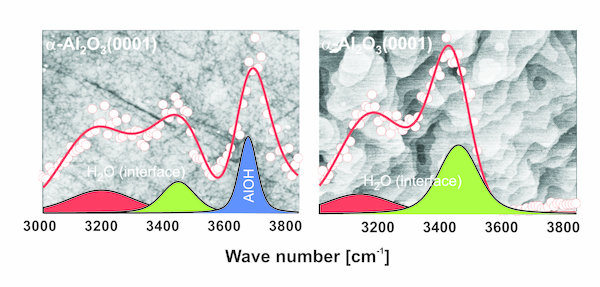
- Oxide/electrolyte interfaces (e.g. SiO2, Al2O3, ZrO2 and Fe2O3)